usp class vi vs fda
Plastics were assigned Class I-VI based on the biological in vivo testing systemic injection intra-cutaneous and implantation tests. The FDA Office of Regulatory AffairsUSP Cooperative Research and Development Agreements enable USP and FDA to collaborate on protocols and work plans that impact the effective development of up-to-date monographs and nomenclature.

028 Epdm 70 Duro White Fda Peroxide Cured Usp Class 6 O Ring Seal Design Store
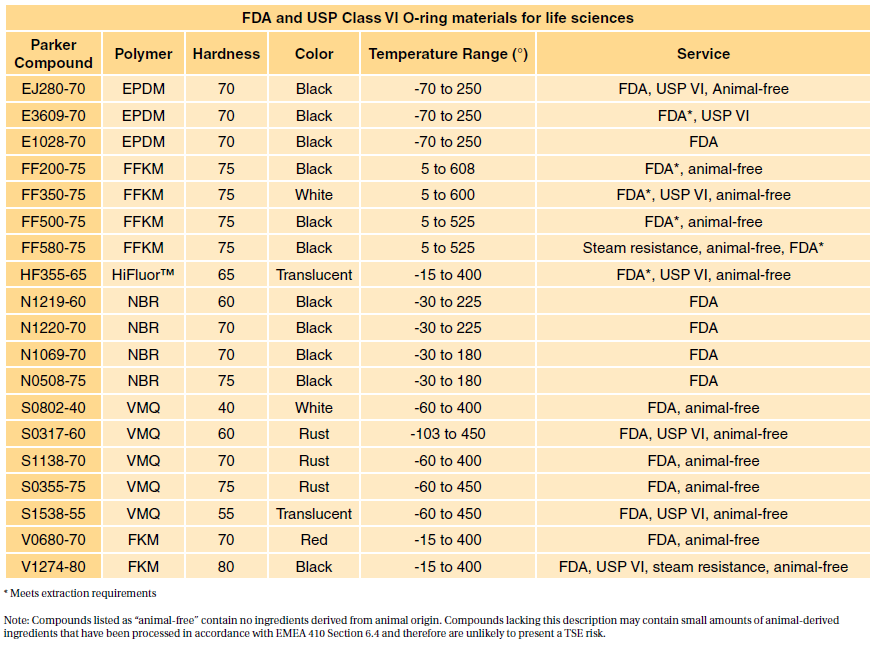
Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant.
. Darcoid and Parker offer a wide range. Among all USP classes Class VI materials meet the most stringent testing requirements. USP Plastic Class VI as this group is also known includes silicones that have passed a systemic toxicity test an.
Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. Specially formulated for long term sealing. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
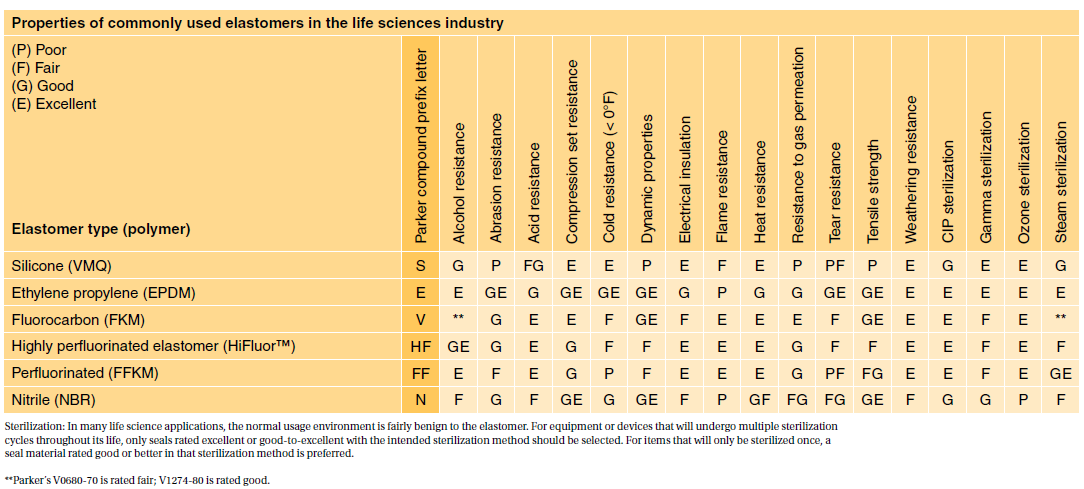
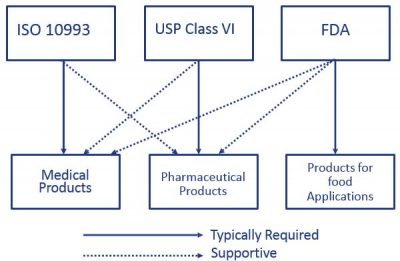
Absorbable Polyhydroxybutyrate Surgical Suture Produced by Recombinant DNA Technology. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products.
USP Class Testing standards are determined by the United States. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. The United States Pharmacopeia USP is a non-governmental not-for-profit public health organization that is an official public standards-setting authority for all prescription and over-the-counter medicines and other health care products manufactured or sold in the United States.
The FDA requires testing of finished devices however the demonstration of biocompatibility of materials according to USP Class VI standards is provided as an aid to device manufacturers in their. Most importantly use of Class VI certified materials substantially reduces the risk of causing harm or increased stress to a patient from reaction to a toxic material. Materials that meet USP Class VI standards generally ensure a high quality level and better acceptance with the FDA and USDA because the materials are believed to substantially reduce the risk of causing harm to patients from reaction to a toxic material.
Typical applications for our FDA NSF 51 USDA materials are disposable medical. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600.
FDA and USP work together to identify areas for monograph or general chapter development where there is a need for. While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993. To begin the USA food and Drug Administration FDA places regulations on three different types of food additives- direct secondary direct and indirect food additives.
FDA and USP Class VI O-Rings. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. Sil 714002 USP class VI Silicone 1 70 Yes transl.
USP Class VI Testing is only one standard of biocompatibility however. Sil 714002 USP class VI Silicone 1 70 Yes transl. The USP also establishes standards for food ingredients.
Depending on the curing method compounders can supply medical injection molders with elastomers that meet FDA regulations and USP Class VI requirements. In order to pass the Class VI standards the productmaterial must exhibit a very low level of toxicity by passing all the tests requirements when tested according to ISO 10993. RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials.
Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. There may be some confusion between FDA USP Class VI and FDA food grade.
Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. Overview of Medical Products Medical grade plastics include materials with either an FDA approval andor USP Class VI approval. It generally ensures a high quality level and better acceptance with the FDA and USDA.
Sil 714001 USP class VI Silicone 1 70 Yes transl. USP Class VI Testing is only one standard of biocompatibility however. That being said if you cant get an ISO 10993 compliant material often because the material simply hasnt been tested using a USP Class VI material is a less risky option.
27 rows The US. The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. FDA food-grade rubber materials typically comply with FDA 21 CFR 1772600 Rubber Articles Intended for Repeated Use.
Specially formulated for long term sealing. Class VI materials which were discussed earlier are tested according to the above protocols. While it is possible a USP Class VI material.
USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. There are six classes VI being the most rigorous. There may be some confusion between FDA USP Class VI and FDA food grade materials.

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Fda And Usp Class Vi O Rings Guide 2020 Nes

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

0 75 3 4 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated

What Is Usp Class Vi Testing Tbl Plastics

Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact
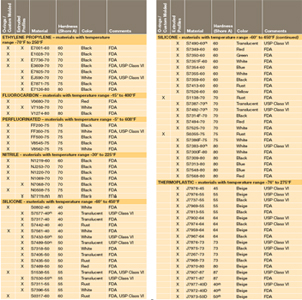
Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Pin On Medical Grade Silicone Tube

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Usp Class Vi Foster Corporation
![]()
Usp Class Vi Silicone Is Independently Certified For Biocompatibility Specialty Silicone Products Inc